3 min read
Why Value-Added Engineering Services Unlock the Value of Advanced Material Enhancements
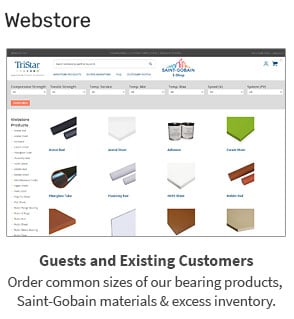
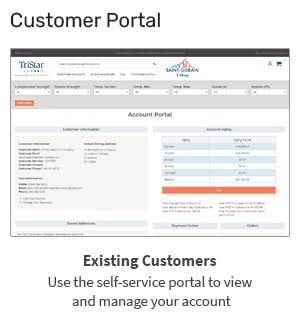
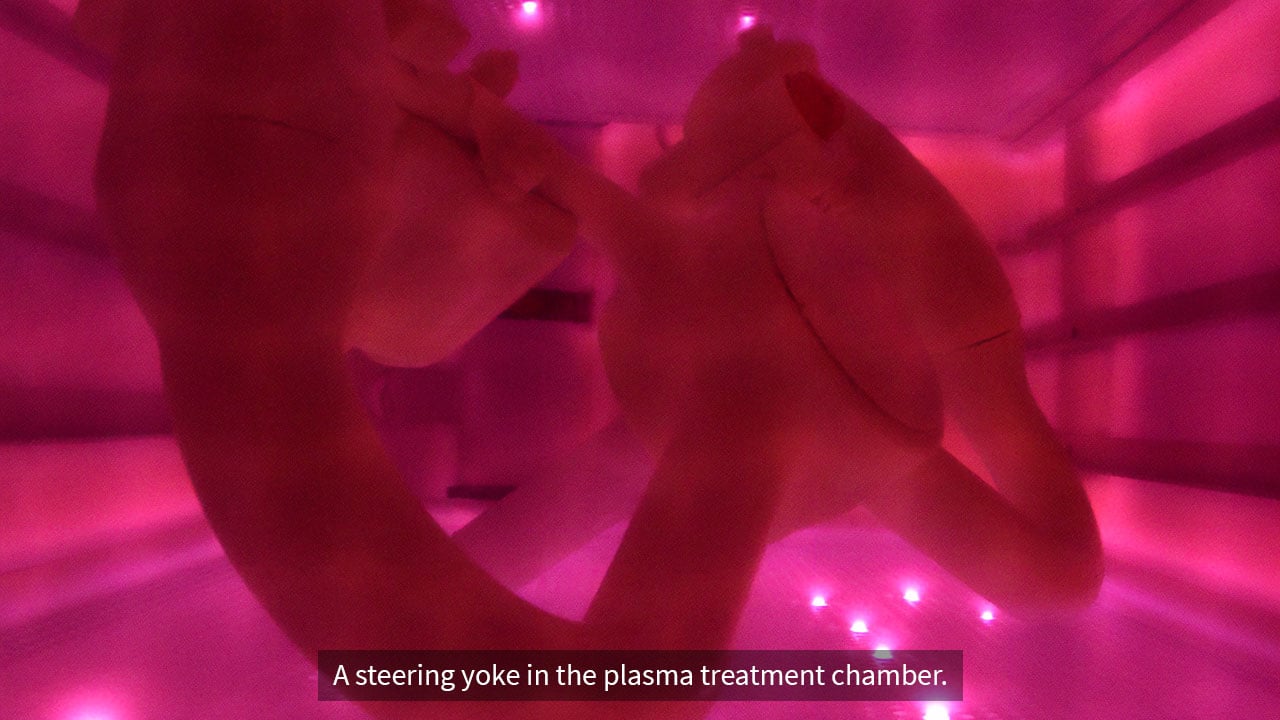
TriStar’s Enhanced Materials Division (EMD) offers advanced technologies like plasma surface treatment and specialized polymer filtration membranes. While these capabilities are incredibly valuable in the right applications, they represent only one...
Read More